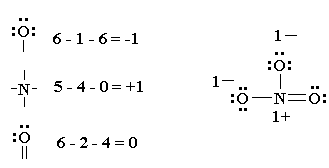Impressive Tips About How To Draw Lewis Diagrams
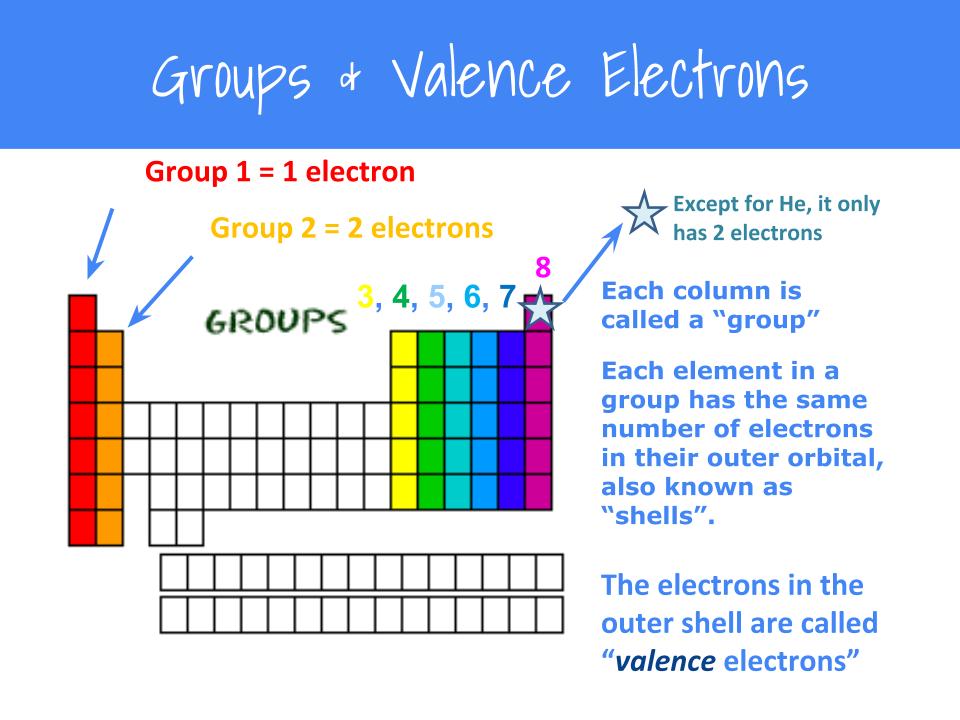
First of all, we need to work out the number of valence electrons in this molecule, ammonia.
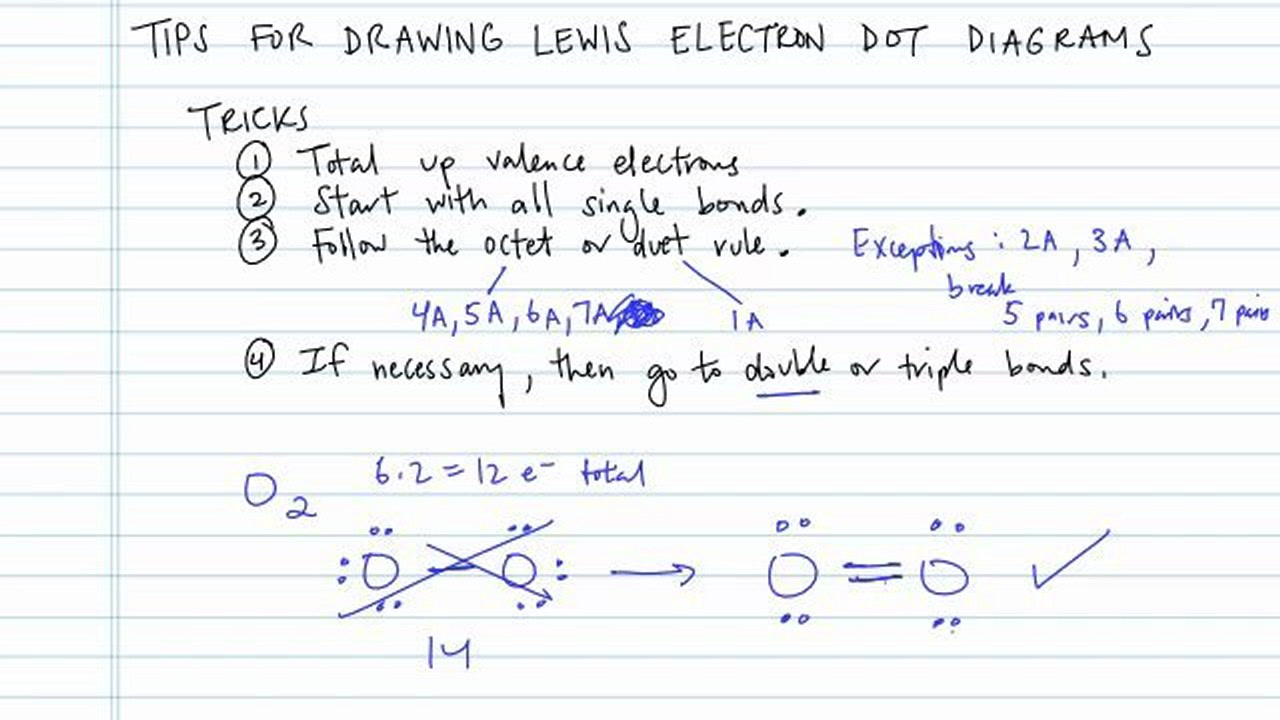
How to draw lewis diagrams. This is a videoscribe tutorial of how to draw lewis diagrams for elements and simple molecules. How to draw lewis dot structures. Steps to draw lewis structure the steps to draw the lewis structures of various types of compounds are given below:
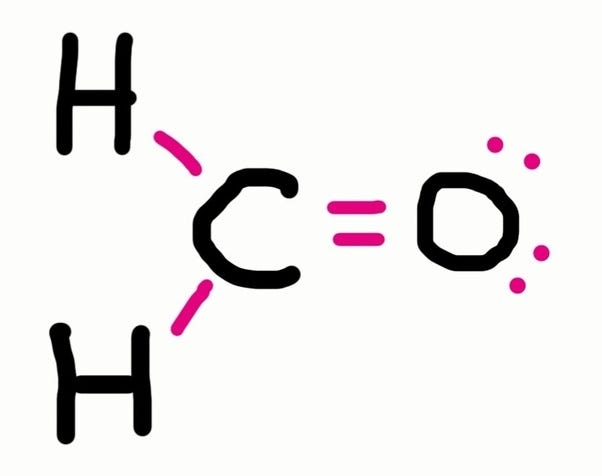
Draw a lewis dot diagram for ammonia, nh 3. (put least electronegative element in the center. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule.
They also display the total number of lone pairs present. 1 chemical formula total number of valence electrons lewis dot. Check your understanding of lewis diagrams in this set of free practice questions designed for ap chemistry students.
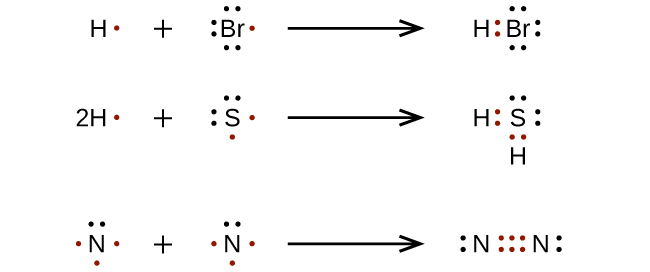
Andersen shows you how to draw lewis dot diagrams for atoms and simple molecules.intro music atributiontitle: Place electron pairs around each. Draw skeletal structure of compound showing what atoms are bonded to each other.
Add your bonds to the drawing. Thus, a pair of dots represent the bond between the draw lewis dot diagrams for the following molecules: Put the least electronegative atom in the center.
(or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the. For a single bond, you would simply draw 1 line from the first atom to the second. We can do this by looking at the periodic table.




/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)